• Research Highlight
Depression signs will be exhausting to handle, particularly for folks with treatment-resistant despair, a persistent and extreme type of the dysfunction. Fortunately, new therapies are rising for such hard-to-treat situations. One of those is the remedy ketamine, which quite a few NIMH-funded research have proven has fact-acting and lasting results in folks with temper problems like despair.
The discovery of ketamine has been a recreation changer for folks with extreme despair, who typically want fast aid from life-threatening signs. Whereas most antidepressants take weeks or months to work, ketamine works inside hours to strongly scale back despair signs in folks for whom different remedies haven’t labored.
Despite ketamine’s effectiveness as an antidepressant, critical issues restrict its use, together with problematic unintended effects and a excessive danger of misuse. To tackle these issues, the National Institutes of Health (NIH) is invested find medicines that capitalize on the therapeutic results of ketamine whereas avoiding its damaging ones.
What did the researchers take a look at within the examine?
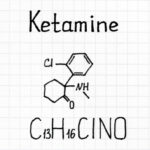
New analysis funded by the NIH Blueprint Neurotherapeutics Network for Small Molecules program examined a novel ketamine-related remedy often called RR-HNK. RR-HNK is a metabolite , or byproduct, of ketamine left over because the physique breaks it down. RR-HNK confirmed antidepressant results in preclinical research with animals , however it had not but been examined in people.
The examine concerned a broad collaboration of researchers within the intramural applications at NIH’s National Institute of Mental Health, National Center for Advancing Translational Sciences, and National Institute on Aging; the Duke University and the University of Maryland School of Medicine; and different nationwide and worldwide establishments.
What did the researchers do within the examine?
This examine examined the protection, tolerability, pharmacokinetics (how the drug strikes by the physique), and pharmacodynamics (how the drug impacts the physique) of RR-HNK for the primary time in people.
Participants had been wholesome adults between 18–65 years. A complete of 74 folks participated throughout three randomized trials: 55 obtained RR-HNK and 19 obtained an inactive placebo. Both the remedy and placebo got intravenously, with individuals and researchers unaware of which group the individuals had been in.
- In Trial 1, individuals obtained one in all six dose ranges of RR-HNK a single time.
- In Trial 2, individuals obtained one in all two dose ranges of RR-HNK 4 occasions over 2 weeks.
- In Trial 3, individuals obtained a single dose of RR-HNK, and their cerebrospinal fluid (a liquid surrounding the mind and spinal wire) was collected.
The examine’s main intention was to find out if the remedy is secure by first testing it in wholesome adults and not using a recognized psychological well being situation. Throughout the examine, the researchers intently monitored for antagonistic occasions, equivalent to damaging unintended effects. The complete security profile included bodily examinations; laboratory outcomes; important indicators; electrocardiograms of coronary heart exercise; and rankings of temper, suicide danger, and dissociation and sedation signs. Additionally, individuals had been requested to inform examine workers in the event that they skilled any issues or unintended effects at any level.
The researchers additionally collected blood and urine samples from all individuals earlier than, throughout, and after receiving the remedy. These samples and the cerebrospinal fluid collected in Trial 3 had been used to verify whether or not the remedy entered the physique and the mind.
As a last exploratory step, the researchers used mind imaging to look at individuals’ gamma oscillations (a kind of mind wave) earlier than and after the remedy. This measure of the mind’s response to stimuli is without doubt one of the few accessible biomarkers of a medicine’s results on the mind.
What did the examine discover?
RR-HNK revealed itself to be exceptionally secure, inflicting no critical antagonistic occasions and solely gentle unintended effects that resolved rapidly with out care. Participants additionally reported no signs of sedation or dissociation. The optimistic security profile was maintained in any respect doses examined and after a number of doses. Together, the outcomes point out that RR-HNK is secure and tolerable, with restricted abuse or misuse potential.
Cerebrospinal fluid confirmed that RR-HNK entered the mind and remained at detectable ranges a number of hours after administration. Results additional confirmed a dose-proportional response to the remedy, that means that at greater ranges of RR-HNK, the quantity of the substance within the physique additionally elevated on the similar price. A predictable relationship between the quantity of RR-HNK given and the quantity of RR-HNK within the bloodstream is essential for the scientific efficacy of the remedy, permitting medical doctors and researchers to precisely calibrate doses to an individual’s particular stage and kind of signs.
In the check of mind exercise, some individuals who obtained low to average doses of RR-HNK, however not those that obtained excessive doses or the placebo, confirmed a rise within the energy of gamma oscillations. Preliminary proof that RR-HNK produces a change in mind exercise strengthens the case for its use as an antidepressant and gives a scientific biomarker for measuring whether or not it really works in future analysis. However, there was giant variability within the outcomes and, given the small variety of individuals, the researchers warning in opposition to drawing agency conclusions from these findings.
What do the outcomes imply?
This examine provides crucial perception into the protection, tolerability, and results of RR-HNK in a various inhabitants of wholesome adults. Findings from this early stage examine display that the ketamine metabolite doesn’t trigger ketamine’s damaging unintended effects and is secure for use in people. The outcomes additionally assist set dosing parameters for its use in future analysis and remedy.
These knowledge, notably a robust security profile, assist the development into the subsequent section of analysis aimed toward creating new therapies for folks with hard-to-treat psychological problems. Despite the small dimension of every trial, which makes it troublesome to interpret a few of the outcomes, the findings maintain promise for the way forward for psychological well being remedy. This examine is a crucial early step in NIMH’s mission to enhance the remedy of psychological sicknesses by analysis, setting the stage for scientific trials that check whether or not RR-HNK successfully treats despair and different problems.
Reference
Raja, S. M., Guptill, J. T., Mack, M., Peterson, M., Byard, S., Twieg, R., Jordan, L., Rich, N., Castledine, R., Bourne, S., Wilmshurst, M., Oxendine, S., Avula, S. G. C., Zuleta, H., Quigley, P., Lawson, S., McQuaker, S. J., Ahmadkhaniha, R., Appelbaum, L. G. … & Thomas, C. J. (2024). A Phase 1 evaluation of the protection, tolerability, pharmacokinetics and pharmacodynamics of (2R,6R)-hydroxynorketamine in wholesome volunteers. Clinical Pharmacology & Therapeutics, 116(5), 1314–1324. https://www.doi.org/10.1002/cpt.3391
Grants
R01MH107615 , ZIAMH002857 , ZIATR000042 , ZIAAG000297
Clinical trial
NCT04711005